…but to truly succeed in today’s fast-paced, cost-conscious environment, projects must be built with top quality while remaining cost-effective.
The High Stakes of Capital Investment
In today’s economic landscape, the pressure to ensure a strong return on capital investment has never been greater. Large-scale capital projects—particularly in industries like energy, technology, and pharmaceuticals—require not only significant financial commitment but also rigorous quality control to minimise risks, be cost effective, accelerate timelines, and maximise long-term value.
Project overruns, delays, and operational inefficiencies due to poor quality are not acceptable. For years, the construction sector has struggled with quality issues in the field, leading to commissioning delays and, ultimately, facilities with poor operability and reliability. However, these costs could be significantly reduced if the industry was to embrace the concept of ‘quality assurance‘ that has been used with great success by other sectors of the industry.
The Missing Link: Quality Assurance in Construction
While industries benefit from well-established design and engineering principles, even the best-designed facilities can fail if construction quality is poor. Designing quality into a facility and embracing the concept of ‘Quality by Design’ (QbD) is essential to achieving high standards.
Many project delivery methods involve breaking a design into manageable packages that are either self-performed or subcontracted. While self-performed work may maintain a predictable level of quality, subcontracted work varies greatly depending on contractor selection. Additionally, the cultures of Good Engineering Practice (GEP) and Good Documentation Practice (GDP) are critical to ensuring consistent quality across all project phases.
Unlike engineering firms that emphasise “Quality by Design” (QbD), construction companies tend to be very cost and time driven, and, therefore, their focus is on task completion and safety. Ideally, field safety and quality should be combined to deliver projects with zero accidents and zero defects. Indeed, many construction companies do not have a quality manual/programme and often fail to see the intrinsic link between quality and safety.
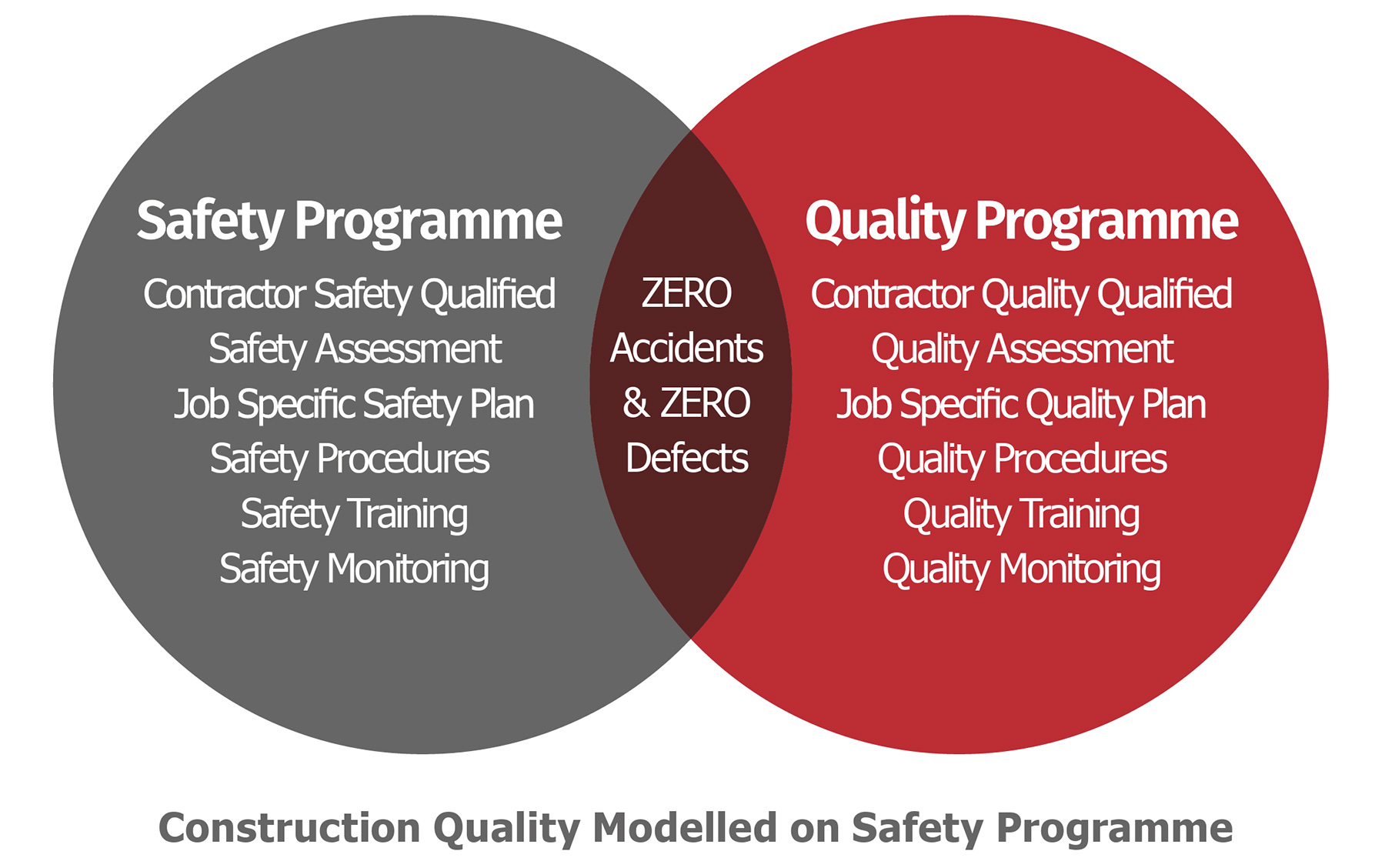
The Role of Construction Quality Assurance (CQA)
At the outset of a project, the level of quality required for each phase must be clearly defined. While this is typically done for the engineering design phase, the critical construction and commissioning phases are often overlooked—despite their significant impact on operability and reliability.
A well-planned commissioning program with detailed protocols and checklists is essential, but it is of little value if the facility is littered with defects. To mitigate this risk, a comprehensive Construction Quality Assurance (CQA) plan should be established early, defining clear quality standards and ensuring alignment among all stakeholders.
Why Third-Party Quality Assurance Makes Sense
Establishing a culture of quality in construction is challenging due to the transient nature of construction teams and the industry’s economic volatility. Unlike engineering firms with long-term stability, construction teams often dissolve after project completion, making long-term quality initiatives difficult to sustain.
A logical solution is to engage a third-party quality management firm that understands operational needs and can integrate quality into the construction process. A quality & commissioning firm with in-depth expertise in field execution and quality assurance can serve as a Construction Quality Assurance (CQA) manager—ensuring a seamless transition from construction to commissioning while keeping costs and timelines under control.
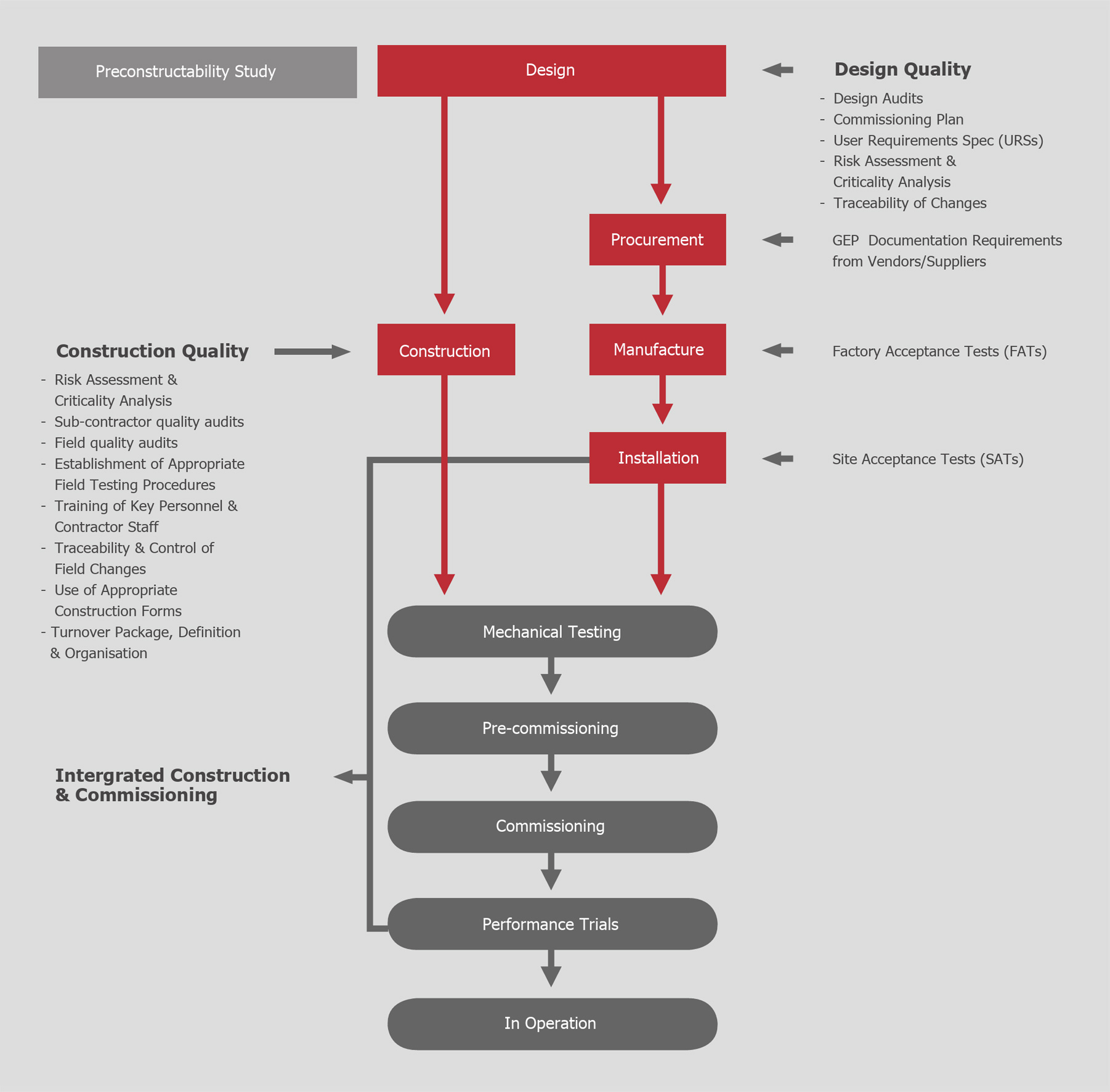
Implementing a Successful CQA Program
A robust CQA program should align with Good Engineering Practices (GEP) and Good Documentation Practices (GDP) to deliver a defect-free, operationally ready facility. Key steps in the program include:
1. Risk Assessment & Criticality Analysis
Identify and prioritize high-risk systems that impact cost and schedule, considering both engineering and field execution perspectives.
2. Subcontractor Assessments
Audit subcontractors’ quality systems, commissioning plans, and processes to address potential deficiencies early.
3. Field Inspections & Reporting
Conduct compliance audits to identify and resolve construction-quality issues before they affect commissioning.
4. Establishment of Field Procedures
Define clear testing protocols, inspection plans, and documentation standards to ensure quality consistency.
5. Traceability & Control of Field Changes
Manage field modifications rigorously to avoid compromising safety, operability, or project timelines.
6. Use of Appropriate Construction Forms
Ensure that all checklists and test forms align with GEP requirements to facilitate commissioning.
7. Turn-Over Package (TOP) Organization
Develop a structured approach to documentation handover, ensuring a smooth transition from construction to operations.
8. Training of Personnel
Foster a ‘right-first-time’ culture through training in GDP and field-quality procedures for all key stakeholders.
The Bottom Line: Quality Equals Efficiency
A well-executed CQA program not only ensures smooth project turnover but also enhances long-term facility performance. By integrating construction quality with commissioning from the outset, owners can reduce delays, lower costs, and accelerate time to market—all while ensuring operational reliability and safety.
Ultimately, selecting a strong construction firm is only half the battle. Bringing in a third-party CQA expert early in the process can have a transformative impact on project success. At the end of the day, a successful project is one where a facility reaches optimal operation in the shortest timeframe—maximising return on investment while ensuring safety and reliability.
Interested in learning more? Contact us today to find out how we can help with your technical needs.
